Anna Kourti’s Group
current projects:
1. Diapause and photoperiodic
clocks.
Diapause is a period of endocrine-mediated metabolic and developmental
arrest induced by changes in abiotic cues that indicate the onset of
adverse environmental conditions, such as winter. This arrest occurs in a
species-specific life stage, and in adult diapause, reproduction halts.
Induction of this arrest typically stimulates nutrient/metabolic storage,
so that a low level of metabolic activity can be maintained and sufficient
reserves are left to continue development or reproduction. Diapause has
consequences for growth, reproduction, survival, and longevity. A glimpse
of the molecular mechanisms underlying diapause has begun to emerge in a
number of insect species, providing us with tantalizing directions for
future research, such as aging. From a genetic perspective, it appears that
genes involved in clock function, insulin signaling, stress
resistance, and development have been co-opted into insect
diapause pathways.
Clock function: All eukaryotes, and some prokaryotes,
have evolved a circadian clock that is set by light to time various daily
activities at the biochemical, physiological and behavioral levels. Some of
these organisms have evolved photoperiodism,
a response to the length of day or night for the timing of development,
reproduction and diapause in anticipation of seasonal changes in the
environment. Bünning first proposed the
functional involvement of the circadian clock into the photoperiodic clock
for measuring the length of day or night.
Circadian clocks are molecular time-keeping mechanisms
that reside in a diverse range of cell types in a variety of organisms.
Since both circadian rhythms and photoperiodism
rely upon daily cycles of environmental change, it seems reasonable to
assume that the same clock elements are involved in both processes.
Photoperiodic clocks allow organisms to predict the
coming season. In insects, the seasonal adaptive response mainly takes the
form of diapause. Though our knowledge of the molecular details of the
circadian clock has advanced rapidly, the functional elements of the
photoperiodic clock remain largely unknown.
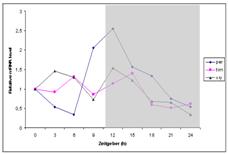
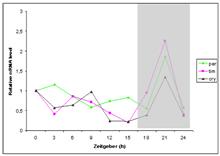
Figure 1. The regulation of S. nonagrioides
per, tim, cry genes through LD 16:8 and
SD 10:14 conditions; Dark period is indicated with light grey (Unpublished
results).
2. New Methodologies for Insect Pest Control by using RNAi
Biopesticides are certain types of pesticides derived from natural materials as animals, plants, bacteria, or certain minerals. Biopesticides are often effective in very small quantities and often decompose quickly, thereby resulting in lower exposures and largely avoiding pollution problems caused by the conventional pesticides. When used as a component of Integrated Pest Management (IPM) programs, biopesticides can greatly decrease the use of conventional pesticides, while crop yields remain high. The feasibility of using RNA interference, (RNAi) in the protection of crops against insect herbivores has been demonstrated. RNAi already proved its usefulness in functional genomic research on insects, but it also has considerable potential for the control of insect pests. Despite having been considered for many years, application of RNAi technology to give plant resistance to herbivorous insects has only just been realised. This approach holds great promise for the future because it allows a wide range of potential targets for suppression of gene expression in the insect to be exploited. The key of RNAi approach success for insect pest control is mainly dependent on careful target selection and a convenient delivery system. The delivery system of the RNAi triggering molecule to the insect pests could be achieved via genetically modified plants, recombinant bacteria, nanoparticles, food traps or direct injection into the insect’s body. We aim to exploit new methods for the control of agricultural insect pests, by using molecular approaches like RNAi. The idea is to assemble the sequencing data of specific insectin contigs and assemble these in a database. We can use to carry out BLAST searches using as queries RNAi target genes that were identified fromspecial insect. We could do BLAST searches on a database of contigs from the transcriptome. 2. Reveal unexploited genes supporting us with information regarding: Pesticide resistance, chemical control, evolution, insect diversification, endocrinology and molecular biology of these two insect pests, 3. Isolate RNAi targets with potential insecticidal properties. After transcriptome sequencing, stage specific genes will be isolated and screened using RNAi bioassays, in order to construct: a. Bio insecticidal/ pesticidal formulations for future applications in pest control, concerning: i. the specific genes as target genes for an RNAi-based pest management, and ii. insecticidal/ pesticidal formulations using the in vitro synthesized dsRNAs.
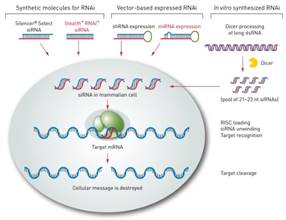
Fig. 1. The mechanism of RNAi
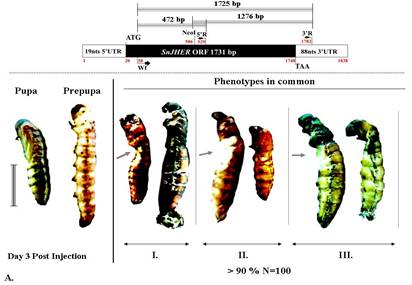
Fig. 2. RNAi using the gene SnSnJHER of Sesamia nonagrioides. SnSnJHER 472 bp or SnJHER 1276 bp or SnJHER 1725 bp after injection in Sesamia nonagrioides larvae L6d9 of dsJHERs. Left: Normal pupa and larva. Right: Stages of pre-pupa and pupa after injection dsJHER472 II. dsJHER1276 ΙΙΙ. dsJHER1725 .
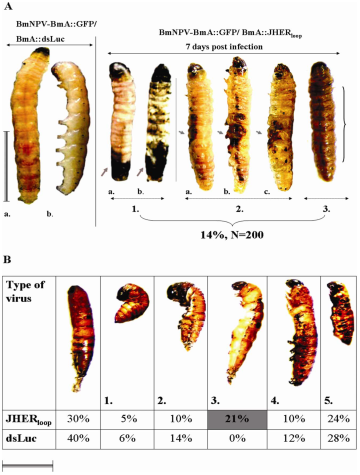
Fig. 3. Abnormal development of L5d3 larvae after injection dsJHER1725 .i. Normal larva, ii. Abnormal larva. iii. Abnormal larva (lateral). iv. Abnormal larva (lateral).