PMG Verticillium dahliae metabolite library v.01 (Last update 21/06/2020)
Showing iCard for L-Tyrosine (3TMS) (PMG-VD0000020)

|
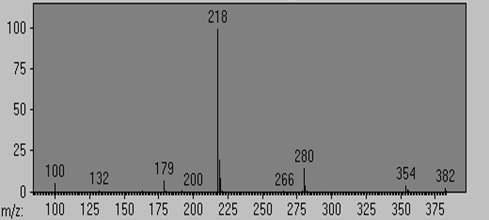
Mass spectrum (Rel abundance vs m/z) |
|
|
Peaks |
( 86 4) ( 91 4) (100 56) (115 5) (132 11) (163 11) (165 4) (174 6) (179 73) (180 15) (181 5) (192 14) (200 2) (203 7) (218 1000) (219 197) (220 89) (221 13) (223 3) (265 11) (266 4) (279 9) (280 149) (281 39) (282 15) (354 40) (355 18) (356 8) (382 30) (383 11)
|
|
Average Retention time (min) |
31.16
|
|
Quant mass (Da) |
218
|

|
Common Name |
L-Tyrosine
|
|
Structure |
|
|
Synonym(s) |
(S)-3-(p-Hydroxyphenyl)alanine; (S)-2-Amino-3-(p-hydroxyphenyl)propionic acid; Tyrosine
|
|
KEGG ID |
|
|
Golm Metabolome Database ID |
A194002
|
|
PubChem CID |
6057
|
|
CAS Registry Number |
60-18-4
|
|
Biosynthetic pathways (KEGG) |
map00130Ubiquinone and other terpenoid-quinone biosynthesis map00231Puromycin biosynthesis map00261Monobactam biosynthesis map00350Tyrosine metabolism map00360Phenylalanine metabolism map00400Phenylalanine, tyrosine and tryptophan biosynthesis map00401Novobiocin biosynthesis map00460Cyanoamino acid metabolism map00680Methane metabolism map00730Thiamine metabolism map00940Phenylpropanoid biosynthesis map00950Isoquinoline alkaloid biosynthesis map00965Betalain biosynthesis map00966Glucosinolate biosynthesis map00970Aminoacyl-tRNA biosynthesis map01063Biosynthesis of alkaloids derived from shikimate pathway map012102-Oxocarboxylic acid metabolism map01230Biosynthesis of amino acids
|
|
Monoisotopic mass (Da) |
181.0739
|
|
Average Molecular Mass (Da) |
181.1885
|
|
Molecular Formula |
C10H13N5O4
|
|
Chemical Group |
Nucleic acids
|

|
Analyzer |
Agilent 6890 MS platform; 5973 series mass selective detector (MSD); 7683 autosampler
|
|
Carrier gas |
Helium |
|
Flow rate |
1 mL min-1
|
|
Column |
HP-5MS, length; 30 m, i.d.; 0.25 mm, film thickness 0.25 μm, Agilent Technologies Inc.
|
|
Ionization |
Electron Impact (EI), Positive
|
|
Mass range |
50-800 Da
|
|
Injector |
5:1 split mode; temperature of 230oC
|
|
Oven temperature |
70oC stable 5 min; 5oC min-1 increase to 295oC; stable 2 min
|
Curator(s): M. LYKOGIANNI, Dr KA ALIFERIS