 | | 1. A Short Biomimetic Approach to the Fully Functionalized Bicyclic Framework of Type A Acylphloroglucinols.
Dakanali, M.; Demadis, K. D.; Vidali, V. P.; Couladouros, E. A.Org.Lett.11(19),4430-4433,2009.[Project infos] | 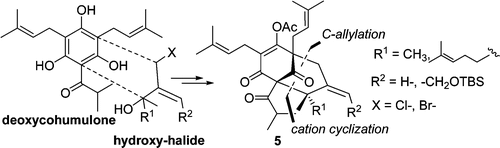 | | Abstract: A biomimetic approach towards type A polyprenylated acylphloroglucinols (PPAPs) is described. The method is based on a C-alkylation – cation cyclization reaction sequence, leading to a convenient build-up of molecular complexity, employing the simple and readily available deoxycohumulone and an appropriately functionalized hydroxy-halide. Thus, a versatile construction of the fully functionalized bicyclic framework of type A PPAPs (5) was achieved | | | 4. Larvicidal activity of naturally occurring naphthoquinones and derivatives against the West Nile virus vector Culex pipiens.
Michaelakis, A.; Koliopoulos, G.; Strongilos, A. T.; Bouzas, E. A.; Couladouros, E. A.Parasitol. Res.104,657-662,2009.[Project infos] | 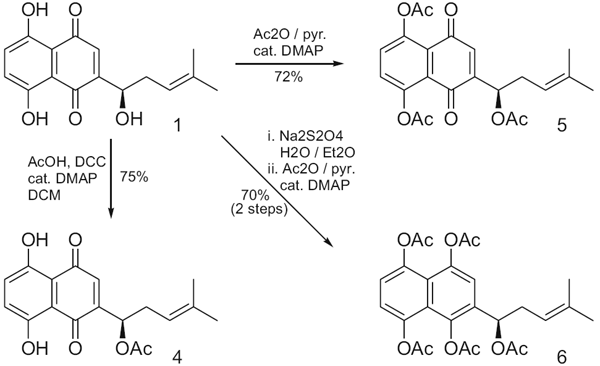 | | Abstract: Concentration-dependent mortality effects were observed for three pure synthetic natural products (alkannin, shikonin, and shikalkin) and three acetylated derivatives of shikonin against Culex pipiens (Culicidae: Diptera) for the first time. The larvicidal properties of all naphthoquinones were evaluated under laboratory conditions against the larvae of the mosquito species C. pipiens biotype molestus, the anthropophilic biotype of the C. pipiens mosquito species. Experimental data of the tested toxicity of quinones revealed generally high efficacy where shikonin (3.9 mg/L) was the most active followed by shikalkin (8.73 mg/L) and alkannin (12.35 mg/L). The insecticidal performance of shikonin-acetylated derivatives was also investigated, aiming at the same time in the establishment of the relationships between the structure and the activity of shikonin-type compounds with larvicidal activity against C. pipiens. Results indicated that naphthoquinones, compared with other natural compounds with larvicidal activity, are very toxic against mosquito larvae and could be a potential source of natural larvicidal substances. Finally, bioassays with shikonin derivatives also revealed that although hydroxylic groups seem to play a secondary role in efficacy, the quinone moiety is essential | | | 5. Open-chain half-bastadins mimic the effects of cyclic bastadins on calcium homeostasis in cultured neurons.
Zieminska, E.; Lazarewicz , J. W.; Couladouros, E. A.; Moutsos, V. I.; Pitsinos, E. N.Bioorg. Med. Chem. Let.18,5734-5737,2008.[Full Article][Project infos] | 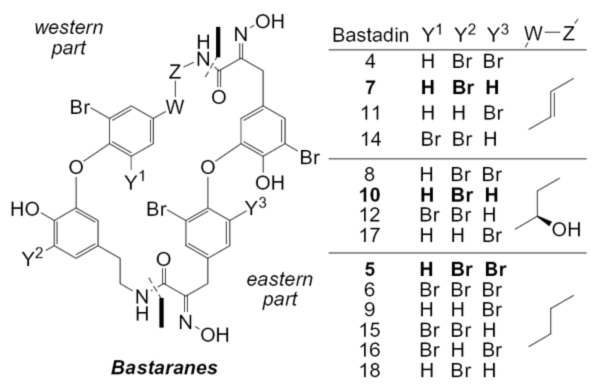 | | Abstract: Constraining the catechol aryl ether moiety of bastadins by incorporation into a macrocycle is not necessary in order to mimic the effects of these marine natural products on neuronal calcium homeostasis. Simple, acyclic analogs that embody the ‘western’ or ‘eastern’ parts of bastadins were found to evoke comparable responses with bastadin 5. | | | 6. Stochastic simulation studies of molecular resists.
Drygiannakis, D.; Patsis, G. P.; Raptis, I.; Niakoula, D.; Vidali, V.; Couladouros, E.; Argitis, P.; Gogolides, E.Microelectron. Eng.84,1062-1065,2007.[Full Article][Project infos] | 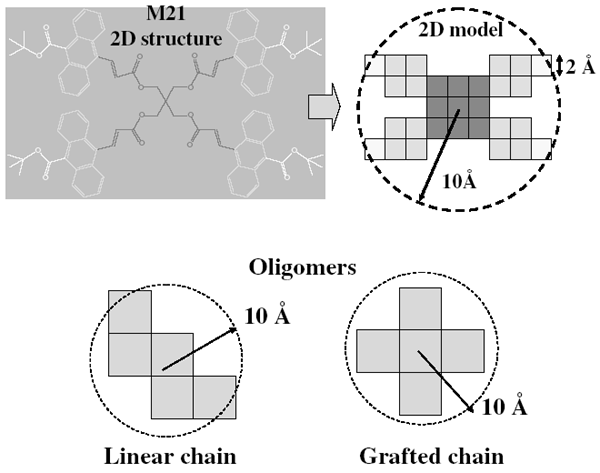 | | Abstract: The influence of resist molecular weight as well as its architecture becomes important in lithographic scales aiming at sub-45 nm resolution. The effects of processing and resist molecular geometry on line-edge roughness (LER) should be well understood in order to meet the ITRS lithographic specifications. In this work, two-dimensional simulations and comparisons of the LER between films of molecular resists and resist films made of oligomers with the same molecular diameter, showed that in all cases molecular resists have lower LER. Explanations of this behavior are proposed based on molecular architecture and the free volume distribution in the resist film. It was also found that the size of free volume regions is less in molecular resist than in the corresponding oligomers. | | | 7. Formal synthesis of the piperidine alkaloid (±)-prosophylline using polymer-supported dihydro-2H-pyridin-3-one.
Couladouros, E. A.; Strongilos, A. T.; Neokosmidis, E.Tetrahedron Lett.48,8227-8229,2007.[Full Article] | 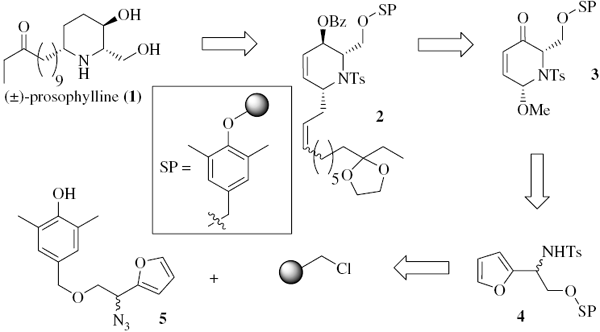 | | Abstract: The use of polymer-supported 2,6-disubstituted-dihydro-2H-pyridin-3-one, as the ‘polymorphic’ core molecule for the formal synthesis of the piperidine alkaloid (±)-prosophylline is presented. | | | 8. A Short and Convenient Chemical Route to Optically Pure 2-Methyl Chromanmethanols. Total Asymmetric Synthesis of b-, g-, and d-Tocotrienols.
Couladouros, E. A.; Moutsos, V. I.; Lampropoulou, M.; Little, J. L.; Hyatt, J. A.J. Org. Chem.5434-5439,2007.[Full Article][Project infos] | 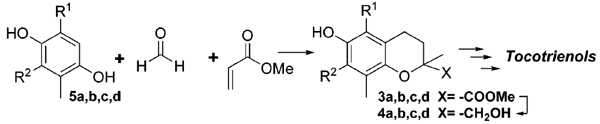 | | Abstract: With use of inexpensive commercially available raw materials, chromanmethanol precursors to the natural a-, a-, and a-tocotrienols have been prepared in high yield. Enzymatic resolution afforded chiral chromanmethanols in high enantiomeric excess. Subsequent attachment of the farnesyl side chain was high yielding, thus allowing the preparation of asymmetric a-, a-, and a-tocotrienols in one final step wherein simultaneous deprotection of the phenol and removal of the sulfone group occurs. This chemistry provides the first synthesis of natural-series a-tocotrienol. | | | 9. Stereostructural Determination by a Synthetic and NMR-Based Approach of Three Oxazinins Isolated from Adriatic Mussels.
Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Grauso, L.; Santelia, F. U.; Tartaglione, L.; Moutsos, V. I.; Pitsinos, E. N.; Couladouros, E. A.Eur. J. Org. Chem.5434-5439,2007.[Full Article] | 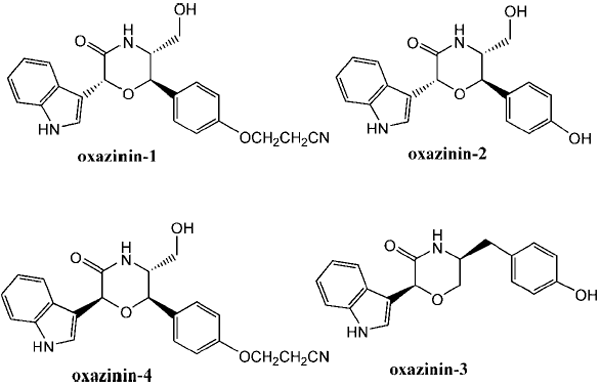 | | Abstract: Two oxazinins, namely oxazinin-5 and -6, along with a related linear precursor (preoxazinin-7) were isolated from toxic mussels collected along the Northern Adriatic coasts in October 2005. Determination of the planar structure of these novel compounds was achieved through extensive NMR spectroscopic analysis, whereas a synthetic approach was crucial for their absolute stereochemical elucidation. | | | 10. Attract-and-kill Strategy. Laboratory Studies on Hatched Larvae of Culex pipiens.
Michaelakis, A.; Mihou, A. P.; Koliopoulos, G.; Couladouros, E. A.Pest Manag. Sci63,954-959,2007.[Full Article][Project infos] |  | | Abstract: The attract-and-kill strategy is a new pest management technique that presupposes the intelligent combination of an attracting agent (e.g. pheromone) and a killing agent (e.g. insecticide). In the present study the potential combination of the microencapsulated synthetic oviposition pheromone, 6-acetoxy-5-hexadecanolide, with an insecticide is tested. Initially polyurea microcapsules containing 6-acetoxy-5-hexadecanolide, the synthetic mixture of diastereomers of the oviposition pheromone of the mosquito species Culex quinquefasciatus (Diptera: Culicidae), have been studied. Laboratory bioassays were performed to confirm the bioactivity of the microencapsulated pheromone on the oviposition activity of Culex pipiens biotype molestus (Diptera: Culicidae) aiming to find out the optimum dose for oviposition response. Its effect showed a dose-dependent manner revealing an optimum dose of 300 mg of dried microcapsules. Attractancy over time was also studied. The microencapsulated pheromone was found to be sufficiently attractive to gravid female mosquitoes for a period of 40 days. Finally, the combination of the synthetic pheromone with the control agent temephos, showed both an acceptable oviposition activity and sufficient larvicidal effect. | | | 11. Prolonged Slow Release of (Z)-11-Hexadecenyl acetate Employing Polyurea Microcapsules.
Mihou, A. P.; Michaelakis, A.; Krokos, F. D.; Mazomenos, B. E.; Couladouros, E. A.J. Appl. Entomol131,128-133,2007.[Project infos] |  | | Abstract: The potential use of polyurea microcapsules, as “release carriers” for insect pheromones, has been demonstrated. (Z)-11-Hexadecenyl acetate (Z11-16:Ac), the major sex pheromone component of several Noctuidae species, was used as model molecule. The coating material ability to release the pheromone was initially studied by the solid phase micro-extraction technique. Polyurea microcapsules released Z11-16:Ac relatively slowly, with a duration of approximately one month, as it was determined under both laboratory and semi-field conditions. Preliminary laboratory bioassays exhibited a satisfactory attraction of Sesamia males, at doses of 50 and 500 mg of dried microcapsules containing the aforementioned pheromone. Almost all male insects tested initiated flight and among them 40.2–49.4% successfully contacted the pheromone local. The preparation of polyurea microcapsules needs further refinement as to increase release duration, nevertheless, these results demonstrate strong potential for the future use of polyurea microcapsules as part of integrated insect control programs. | | | 12. Synthetic Bastadins Modify the Activity of Ryanodine Receptors in Cultured Cerebellar Granule Cells.
Zieminska, E.; Stafiej, A.; Pitsinos, E. N.; Couladouros, E. A.; Moutsos, V.; Kozlowska, H.; Toczylowska, B.; Lazarewicz, J. W.Neurosignals15,283-292,2006-07.[Full Article][Project infos] | 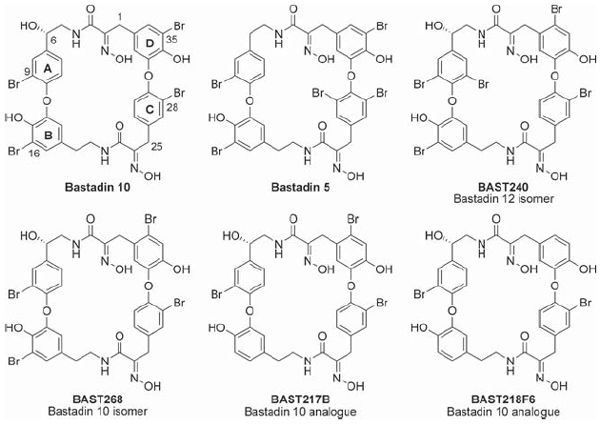 | | Abstract: Although the interactions of several natural bastadins with the RyR1 isoform of the ryanodine receptor in sarcoplasmic reticulum has been described, their structure-dependent interference with the RyR2 isoform, mainly expressed in cardiac muscle and brain neurons, has not been studied. In this work, we examined calcium transients induced by natural bastadin 10 and several synthetic bastadins in cultured cerebellar granule cells known to contain RyR2. The fluorescent calcium indicator fluo-3 and confocal microscopy were used to evaluate changes in the intracellular Ca2+ concentration (Cai), and the involvement of ryanodine receptors was assessed using pharmacological tools. Our results demonstrate that apart from the inactive BAST218F6 (a bisdebromo analogue of bastadin 10), synthetic bastadin 5, and synthetic analogues BAST217B, BAST240 and BAST268 (at concentrations >20 µM) increased Caiin a concentration-dependent, ryanodine- and FK-506-sensitive way, with a potency significantly exceeding that of 20 mM caffeine. Moreover, the same active bastadins at a concentration of 5 µM in the presence of ryanodine prevented a thapsigargin-induced increase in Cai. These results indicate that bastadins, acting in a structure-dependent manner, modify the activity of RyR2 in primary neuronal culture and provide new information about structure-related pharmacological properties of bastadins. | | | 14. Formal synthesis of Abyssomicin C.
Couladouros, E. A.; Bouzas, E. A.; Magos, A. D.Tetrahedron62,5272-5279,2006.[Full Article][Project infos] | 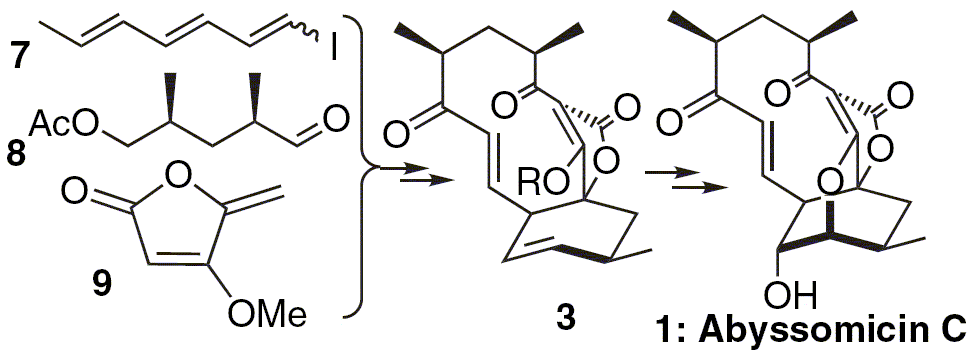 | | Abstract: An alternative strategy towards Abyssomicin C (1) is described. The key ene-diene intermediate is synthesized via a Kishi type coupling of an E/Z mixture of triene-iodide 7 and a suitably functionalized derivative of 2,4-dimethylglutaric acid. A final in situ isomerization/intramolecular Diels–Alder cyclization resulted in the formation of the known intermediate 3 as a single isomer in high yield. Further heating of 3 using excess of iodine, afforded iodo-derivative 23, having the entire carbon skeleton of Abyssomicin D. | | | 15. Oxazinins from toxic mussels: isolation of a novel oxazinin and reassignment of the C-2 configuration of oxazinin-1 and -2 on the basis of synthetic models.
Ciminiello, P.; Dell'Aversano, C.; Fattorusso, E.; Forino, M.; Magno, S.; Santelia, F. U.; Moutsos, V. I.; Pitsinos, E. N.; Couladouros, E. A.Tetrahedron62,7738-7743,2006.[Full Article] | 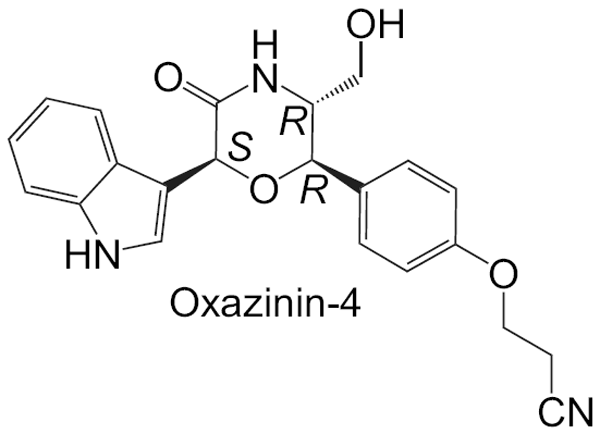 | | Abstract: The analysis of a batch of toxic mussels (Mytilus galloprovincialis) from the Northern Adriatic Sea led to the isolation of a novel oxazinin, oxazinin-4. Its structure including the relative stereochemistry has been elucidated through extensive NMR analysis. A synthetic route to oxazinins has been crucial in establishing the absolute stereochemistry of oxazinin-4 and for reassigning the absolute C-2 configuration of oxazinin-1 and -2 previously isolated from toxic shellfish and stereostructurally characterized. | | | 16. Synthesis and Biological Evaluation of Novel Steroid-Modified Ether Phospholipids.
Karantonis, H. C.; Couladouros, E. A.; Antonopoulou, S.; Pitsinos, M. N.; Demopoulos, C. A.Chemistry and Physics of Lipids138,12-19,2005.[Full Article] | 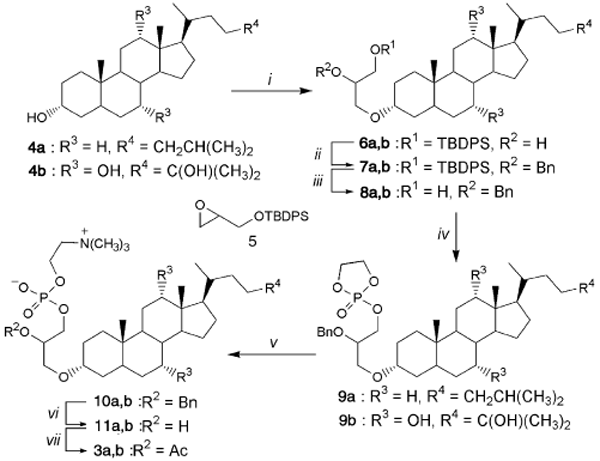 | | Abstract: Platelet activating factor is one of the most potent inflammatory ether phospholipid mediators known and structurally modified analogues are of considerable interest as potential therapeutic preparations. Inspired by the proposed structure for a novel endogenous hydroxy-PAF analogue isolated recently from gingival crevicular fluid, we designed and prepared two novel steroid-modified ether phospholipids. These two novel compounds exhibit marked chemical and biological similarities to their endogenous prototype and they antagonize it being less active in inducing washed platelet aggregation through PAF receptors. | | | 17. Oviposition Responses of Culex pipiens to a Synthetic Racemic Culex quinquefasciatus Oviposition Aggregation Pheromone.
Michaelakis, A.; Mihou, A. P.; Couladouros, E. A.; Zounos, A. K.; Koliopoulos, G.J. Agric. Food Chem.53,5225-5229,2005.[Full Article] | 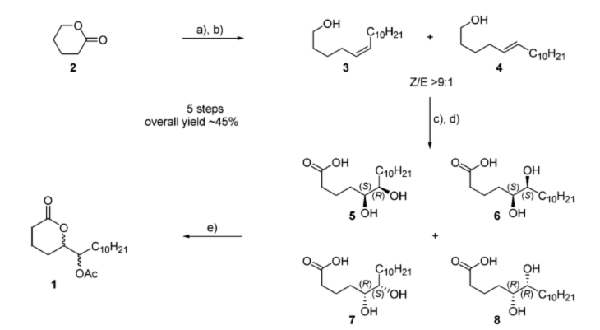 | | Abstract: The oviposition pheromone of Culex quinquefasciatus was synthesized in a racemic form with a simple (five steps), efficient, high yielding (45% total yield) and low cost way (use of relatively low cost reagents). Our Synthetic Racemic Pheromone (SRP) was tested in the laboratory for its bioactivity on Culex pipiens biotype molestus which is a member of the species complex that Culex quinquefasciatus belongs. In the testing conditions it was found bioactivity at the doses of 0.01, 0.1, 1 and 10 µg per cage with best bioactivity achieved at 1µg per cage. The effectiveness of our SRP offers a capable tool for improving mosquito oviposition traps for surveillance or even control programs. | | | 18. A General Method for the Synthesis of Bastaranes and Isobastaranes. First Total Synthesis of Bastadins 5, 10, 12, 16, and 21.
Couladouros, E. A.; Pitsinos, E. N.; Moutsos, V. I.; Sarakinos, G.Chem. Eur. J.11,406-421,2005.[Full Article][Project infos] | 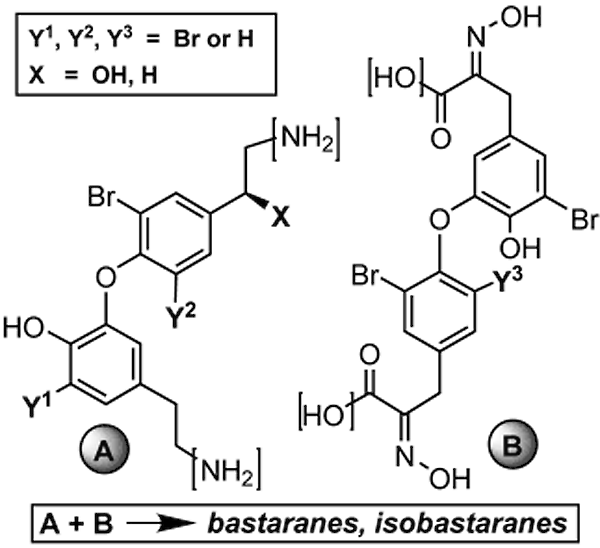 | | Abstract: A general strategy for the synthesis of twenty naturally occurring bastadins (all but bastadin 3) is presented. A key retrosynthetic disconnection of the two amide bonds, common in all target molecules, bisects the macrocyclic core into two diaryl ether fragments, an ,-diamine (western part) and an ,-dicarboxylic acid (eastern part). Efficient preparation of the synthetically challenging o-mono or dibromo-substituted diaryl ether linkages was achieved employing the diaryl iodonium salt method. Regarding the western part, variations of the aliphatic chain were more efficiently secured by the preparation of two different ,-aminonitrile moieties. Cobalt boride mediated reduction of the nitrile functionality established the required diamines and, at the same time, provided the necessary variation of the aromatic-ring bromination pattern. Regarding the eastern part, two different dicarboxyl precursors had to be prepared in order to accommodate bromination-pattern variations. Coupling and subsequent macrolactamization of different combinations of these key intermediates may lead at will to any member of this family of marine natural products. Four bastaranes (bastadins 5, 10, 12 and 16) and two isobastaranes (bastadins 20 and 21) were synthesized as a demonstration of the flexibility and efficiency of the approach presented. | | | 19. Solid Phase Total Synthesis of ()-Phenylahistine and ()-Aurantiamine. Synthesis of a Diverse Dehydro-2,5-diketopiperazine Library. Part 2.
Couladouros, E. A.; Magkos, A. D.Mol. Diver.9,111-121,2005.[Full Article][Project infos] |  | | Abstract: The preparation of solid supported glycine phosphonate and its utilization for the total synthesis of two natural products is presented. The proposed protocol combines diversity with accessibility and speed, which makes this scaffold suitable for automated parallel synthesis and combinatorial chemistry. The preparation of two small libraries of dehydro-2,5-diketopiperazines, combining several natural amino acids with diverse heterocycles (including thiazoles, pyridines, indoles and imidazoles), is also demonstrated. | | | 20. Total Asymmetric Synthesis of ()-Phenylahistine, ()-Aurantiamine and Related Compounds. Part 1.
Couladouros, E. A.; Magkos, A. D.Mol. Diver.9,99-109,2005.[Full Article][Project infos] | 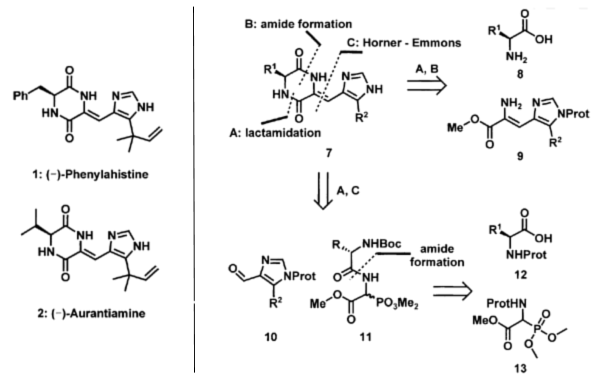 | | Abstract: A new general, short, and efficient strategy for the construction of dehydro-diketopiperazines was developed. Horner – Emmons type coupling between a phosphinyl glycine ester and a formyl heterocycle is the key coupling reaction, which proceeds in good-to-excellent yields on several sterically-hindered substrates. Moreover, racemization of the parent L-amino acids is avoided as a result of the mild basic conditions used. The selection of the NH protective group of the formyl heterocycle was crucial. N-tosylated heterocycles proved ideal for this reaction sequence. Thus, the title compounds, (–)-Phenylahistine and (–)-Aurantiamine, were prepared in high yield (four steps, 47% overall) and optical purity. Furthermore, the synthesis of unnatural derivatives including an indole analogue was successfully completed. | | | 21. Synthetic Studies Towards Oxazinines. An Expedient First Total Synthesis and Proof of the Absolute Stereochemistry of Oxazinin-3.
Couladouros, E.A.; Moutsos, V. I.; Pitsinos, E. N.Tetrahedron Lett.45(41),7779-7781,2004.[Full Article] | 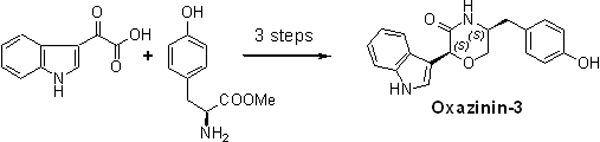 | | Abstract: A synthetic strategy, based on intramolecular addition of an appropriate hydroxyl substituent to a 3-methyleneindolenine, towards the naturally occurring marine toxins oxazinin-1, -2 and -3 is presented. The expedient first total synthesis of oxazinin-3, thus accomplished, demonstrated the efficiency of the approach and established the absolute stereochemistry of oxazinin-3 as 2S,5S. | | | 22. Novel Stereocontrolled Approach to syn- and anti-Oxepene-Cyclogeranyl trans-Fused Polycyclic Systems. Asymmetric Total Synthesis of ()-Aplysistatin, (+)-Palisadin B, (+)-12-Hydroxy-Palisadin B and the AB Ring System of Adociasulfate-2 and Toxicol A.
Couladouros, E. A.; Vidali, V. P.Chem. Eur. J.10(15),3822-3835,2004.[Full Article][Project infos] | 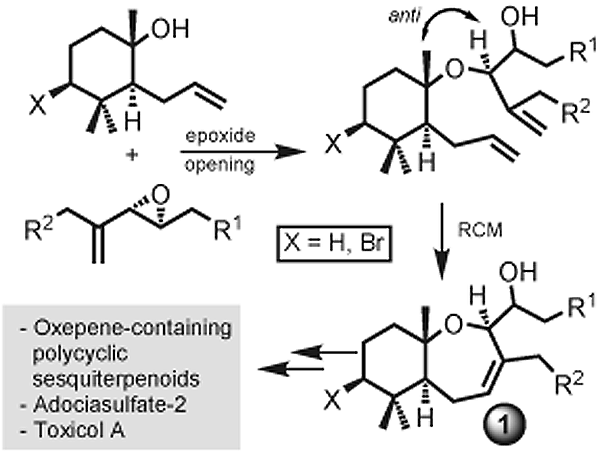 | | Abstract: A new stereocontrolled method for the formation of trans-anti cyclogeranyl-oxepene systems is described. The demanding stereochemistry is secured by stereoselective coupling of a cyclogeranyl tertiary alcohol with a 1,2-unsymmetrically substituted epoxide, while the formation of the highly strained oxepene is achieved employing ring-closing metathesis. Since the stereochemistry of the trans-fused 6,7-ring system is determined by the epoxide, the method also allows the construction of trans-syn 6,7-ring systems. This approach leads to the synthesis of the AB fragment of Adociasulfate-2 and Toxicol A, for the first time. The flexibility and efficiency of the presented strategy is demonstrated by the total asymmetric synthesis of (-)-Aplysistatin, (+)-Palisadin A, (+)-12-hydroxy-Palisadin B, and (+)-Palisadin B, employing two similar key intermediates. | | | 23. First Total Synthesis of cis- and trans-Resorcylide: Remarkable Hydrogen-Bond-Controlled, Stereospecific Ring Closing Metathesis.
Couladouros, E. A.; Bouzas E., Mihou, A. P.Org. Lett.6,977-980,2004.[Full Article][Project infos] | 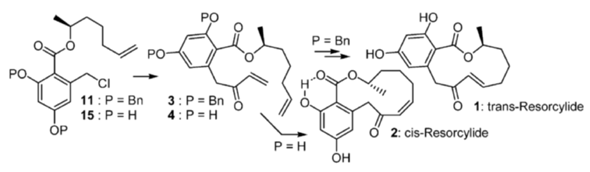 | | Abstract: Stereospecific synthesis of the pair of natural macrolides, trans- and cis-Resorcylide, was performed using ring closing metathesis on dienes 3 and 4, which lack or feature an intramolecular H-bond, respectively. An effective Stille carbonylative coupling of benzyl chlorides 11 and 15 was employed for their preparation. The influence of intramolecular H-bonding on the interconvertions of resorcylides was also studied. | | | 24. Synthesis and Evaluation of Three Novel Scyphostatin Analogues as Neutral Sphingomyelinase Inhibitors.
Pitsinos, E. N.; Wascholowski, V.; Karaliota, S.; Rigou, C.;Couladouros, E. A.; Giannis, A.ChemBioChem4,1223-1225,2003.[Full Article] | 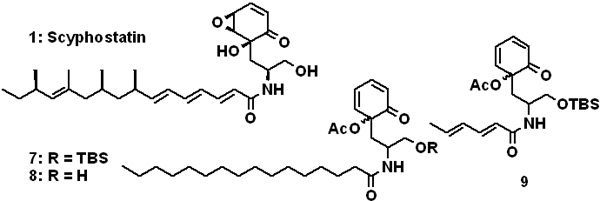 | | Abstract: Three novel analogues of scyphostatin lacking the epoxy moiety were prepared in a short and efficient way starting from 2-allylphenol. Their activity against neutral sphingomyelinase was evaluated leading to conclusions that could contribute in the design and medicinal development of potent and selective inhibitors. | | | 25. Synthesis of o-Halogenated Diaryl Ethers Using Symmetrical Iodonium Salts: Application to the Synthesis of Bastadin precursors.
Couladouros, E. A.; Moutsos, V. I.; Pitsinos, E. N.Arkivoc, (xv)92-101,2003.[Full Article][Project infos] | 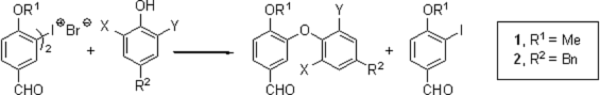 | | Abstract: The coupling of o-brominated phenols with symmetrical iodonium salts for the construction of the corresponding diaryl ethers was studied. The iodonium salt 2, only once mentioned in the literature, was fully characterized and tested for the synthesis of Bastadin related diaryl ethers. | | | 26. Photoresist Etch Resistance Enhancement Using Novel Polycarbocyclic Derivatives as Additives.
Gogolides, E.; Argitis, P.; Couladouros, E. A.; Vidali, V. P.; Vasilopoulou, M.; Cordoyannis, G.; Diakoumakos, C. D.; Tserepi, A.21 (1),Jan/Feb 141-147,2003.[Full Article] | 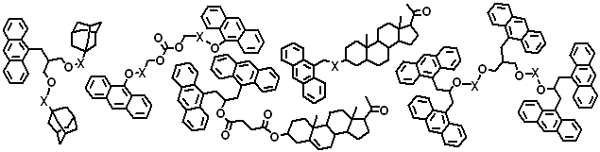 | | Abstract: Novel (in house synthesized) mixed derivatives containing at least two carbocycles per molecule from the group of anthracenes, adamantanes and steroids with functionalized carbon chains are used as modifiers of resist properties and especially etch resistance enhancement and absorption characteristics. Etch resistance enhancement is demonstrated in oxygen, fluorine, and chlorine-containing plasmas under Reactive Ion Etching and Inductively Coupled Plasma etching conditions for Poly(methyl-methacrylate) and a chemically-amplified, positive-tone, methacrylate-based 193nm photoresist formulation. High resolution Experiments show that the proposed additives do not adversely affect the lithographic properties of the photoresists. The additive role is explained with empirical etch parameters such as the Ohnishi number and the Ring Parameter. | | | 27. Synthetic Studies Towards Trichodimerol and Related Vertinoid Polyketides.
Pitsinos, E. N.; Vidali, V. P.; Couladouros, E. A.Arkivoc, (viii)105-110,2002.[Full Article][Project infos] | 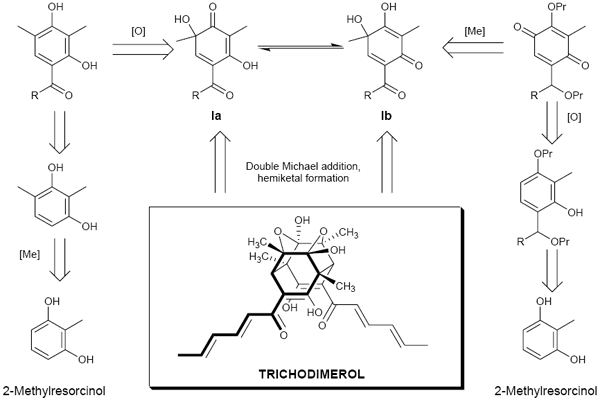 | | Abstract: An alternative synthetic route towards trichodimerol, bisorbicillinol and epoxysorbicillinol employing a suitable para-quinol as a common versatile key synthetic intermediate was investigated. The validity of this approach for the preparation of bisorbicillinol and epoxysorbicillinol derivatives was demonstrated. However, attempts to obtain trichodimerol related derivatives or to render this approach enantioselective were unsuccessful. | | | 28. Generation of Libraries of Pharmacophoric Structures with Increased Complexity and Diversity by Employing Polymorphic Scaffolds.
Couladouros, E. A.; Strongilos, A. T. Angew. Chem. Inter. Ed.41,3677-3680,2002.[Full Article] | 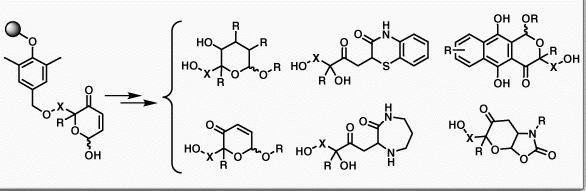 | | Abstract: Antimicrobials, antifungals, anticoccidials, pesticides, and herbicides-druglike scaffolds with potential applications such as these are provided by a combinatorial library containing oxazolediones, naphthoquinones, thiazinones, and diazepinones which were obtained within a few simple steps from solid-supported 2H-pyran-3(6H)-ones as “polymorphic” key intermediate leads | | | 29. Synthesis of Hydroxylated Naphthoquinone Derivatives.
Couladouros, E. A.; Strongilos, A. T.Eur. J. Org. Chem. 3341-3350,2002.[Full Article] | 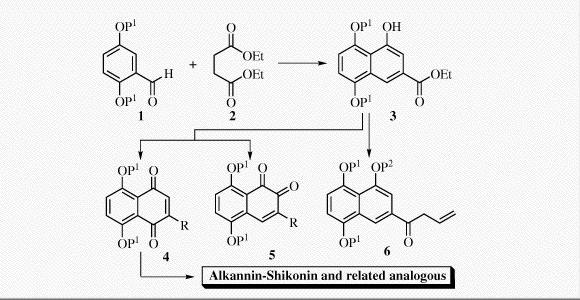 | | Abstract: The use of the Stobbe condensation for the synthesis of juglone derivatives is presented, together with studies towards their further functionalization. The regiospecific oxidation of the above products to o- or p-naphthoquinones was also investigated. Finally, the preparation of useful intermediates for the synthesis of related natural products such as alkannin and shikonin is proposed | | | 30. A New Efficient Route for Multigram Asymmetric Synthesis of Alkannin and Shikonin.
Couladouros, E. A.; Strongilos, A. T.; Papageorgiou, V. P.; Plyta, Z. F.Chem. Eur. J.8,1795-1803,2002.[Full Article][Project infos] | 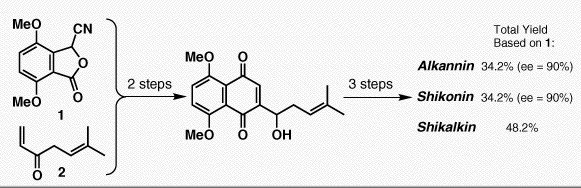 | | Abstract: A short and convergent approach for the synthesis of alkannin, shikonin and shikalkin is presented. A Hauser-type annulation of cyanophthalide 26 with enone 7 affords the complete aromatic system in just one step with concomitant attachment of the entire side chain. Subsequent Corey’s oxazaborolidine mediated asymmetric reduction of the above advanced intermediate, leads to the required isomer in high enantiomeric excess. Finally, a selective and high yielding deprotection protocol furnishes the title compounds as pure crystalline precipitates. Thus, a multigram synthesis of shikonin, alkannin and shikalkin is achieved in high yield and enantioselectivity | | | 32. Biomimetic Total Synthesis of Bisorbicillinol, Bisorbibutenolide, Trichodimerol and Designed Analogues of Bisorbicillinoids.
Nicolaou, K. C.; Vassilikogiannakis, G.; Simonsen, K. B.; Baran, P. S.; Vidali, V. P.; Pitsinos, E. N.; Couladouros, E. A.J. Am. Chem. Soc.122, 3071-3079,2000.[Full Article][Project infos] | 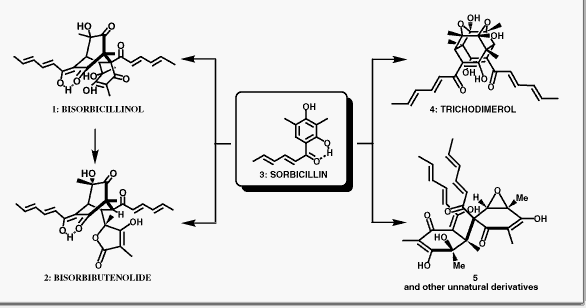 | | Abstract: The bisorbicillinoids are a growing class of novel natural products endowed with unique biological activity and are associated with fascinating hypotheses for their biosynthesis. A full account of our biomimetic explorations toward the bisorbicillinoids including the total syntheses of bisorbicillinol (1), bisorbibutenolide (2), and trichodimerol (4) from sorbicillin (3) is disclosed. Utilizing the novel dimerization reactions discovered and fine-tuned en route to 1 and 4, several analogues of these natural products have been synthesized. Furthermore, studies on the scope of these novel cycloaddition reactions and the isolation of a number of unexpected products along with proposed mechanisms for their formation are reported. These findings add to our knowledge of the largely unexplored chemistry of o-quinols and related aromatic systems | | | 33. Regioselective Oxidation of 3-Monosubstituted Juglone Derivatives.
Couladouros, E. A.; Strongilos, A. T.Tetrahedron Lett.41,535-538,2000.[Full Article] | 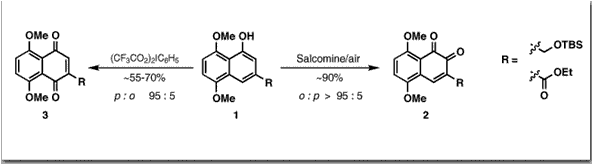 | | Abstract: Derivatives of both ?- and p-naphthoquinones, as well as their corresponding 1,2- and 1,4-dihydroxynaphthalenes, have a wide spectrum of applications in biology, pharmacology and material science, in addition to their use in the chemical and pharmaceutical industry. Furthermore, they are also key intermediates for the synthesis of several biologically active natural products and industrially useful compounds. The most direct synthetic approach for this kind of products is considered to be the regioselective oxidation of the parent juglone derivative. In the present work derivatives of 3-substituted juglones with either electron-withdrawing or –donating substituents are regioselectively oxidized to o- or p- naphthoquinones using salcomine/air or [bis(trifluoroacetoxy)iodo]benzene, respectively. The structure of the oxidation products was confirmed by chemical transformations. A correlation between chemical shift of the single quinoid proton and the quinone structure was established | | | 34. Biomimetic Explorations Towards the Bisorbicillinoids. Total Synthesis of Bisorbicillinol, Bisorbibutenolide and Trichodimerol.
Nicolaou, K. C.; Simonsen, K. B.; Vassilikogiannakis, G.; Baran, P. S.; Vidali, V. P.; Pitsinos, E. N.; Couladouros, E. A.Angew. Chem. Int. Ed.38,3555-3559,1999.[Full Article][Project infos] | 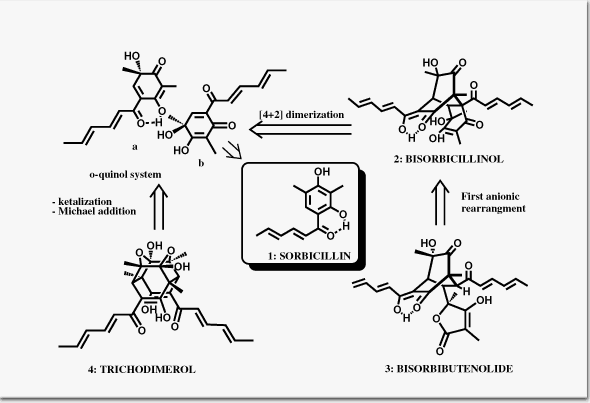 | | Abstract: The findings described in this paper including the total synthesis of bisobicillinol, bisorbibutenolide and trichodimerol shine light on the chemistry of the bisorbicillinoids and confirm a number of hypothetical proposals regarding their biosynthesis. Furthermore, the novel synthetic pathways elucidated during this study add to the repertoire of cascade reactions as an enabling technology for complex molecule construction, while the unusual molecular frameworks produced enrich our pool of advanced synthetic intermediates and building blocks | | | 35. Stabilization of Microtubules by Combretastatin D Derivatives.
Couladouros, E. A.; Li, T.; Moutsos, V. I.; Pitsinos, E. N.; Soufli, I. C.Bioorg. Med. Chem. Lett.9,2927-2928,1999.[Full Article] | 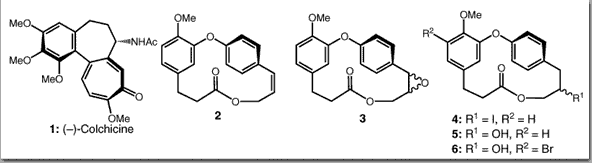 | | Abstract: The effect of some Combretastatin D derivatives on tubulin polymerization at different concentrations was determined by the filtration-colorimetric method. The results showed that all tested compounds interfere with the polymerization of tubulin. However in contrast to colchicine and combretastatins A which inhibit tubulin polymerization, they favor to various degrees the formation of microtubules suggesting a different binding site or mode of interaction for the tested compounds. Derivatives bearing polar substituents were also examined and found to be the most active | | | 36. A General Synthetic Route Towards Bastadins. Part 2: Synthesis of the Western Part of Bastadins 4-16, and Fully Functionalized Macrocycle of Bastadin 12.
Couladouros, E. A.; Moutsos, V. I.Tetrahedron Lett.40,7027-7030,1999.[Full Article][Project infos] | 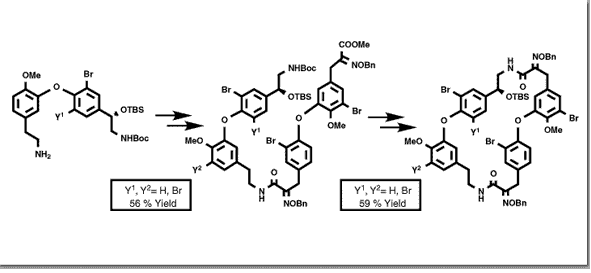 | | Abstract: A general synthetic route for the construction of the western part of the macrocyclic bastadins 4-16 is presented. The western and the eastern segments were coupled using the imidazolide of the corresponding acid. The bromine at position Y2 may be added at this advanced step regiospecifically, strengthening the convergence of the presented approach. Finally, the fully functionalized ?,?-aminoacid is cyclized with EDC affording the macrocyclic ring of bastadin-12 in 72% yield (3.5% overall yield) | | | 37. A General Synthetic Route Towards Bastadins. Part 1: Synthesis of the Eastern Part of Bastadins 4-16.
Couladouros, E. A.; Moutsos, V. I. Tetrahedron Lett.40,7023-7026,1999.[Full Article][Project infos] | 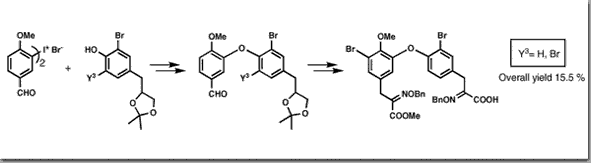 | | Abstract: Bastadins, natural products isolated from the marine sponges Ianthella basta and Psammaplysilla purpurea, are a family of linear or macrocyclic bis-biaryl ether tetrapeptides possessing brominated aryl units and a unique ?-oximino amide bond. In addition to their antibacterial, cytotoxic and anti-inflammatory activity, they constitute a new class of chemical probes for studying immunophilin/ryanodine-sensitive Ca2+ channel interactions in skeletal muscle. A general synthetic route for the construction of the eastern part of the macrocyclic bastadins 4-16 is now presented. The brominated biaryl ethers are synthesized using the iodonium salt method. The synthesis is accomplished within 18 steps in 15.5% overall yield | | | 38. A General Synthetic Route Towards ?- and ?-Lactones. Total Asymmetric Synthesis of (-)-Muricatacin and the Mosquito Oviposition Pheromone (5R, 6S)-6-Acetoxy-hexadecanolide.
Couladouros, E. A.; Mihou, A. P.Tetrahedron Lett.40,4861-4862,1999.[Full Article] | 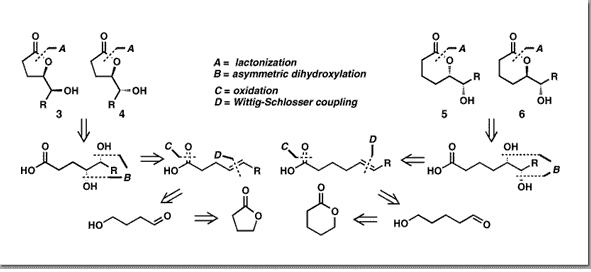 | | Abstract: Five (or six) membered asymmetric lactones are synthesized from ?-butyrolactone (or ?-valerolactone) in a straightforward way using the following reaction sequence: reduction, Wittig-Schlosser coupling, Sharpless asymmetric dihydroxylation, oxidation and lactonization. Thus, (–)-muricatacin is synthesized in six steps (43% overall yield). Furthermore, (5R,6S)-6-Acetoxy-hexadecanolide is prepared in eight steps (38% overall yield) via a carbonate ester, utilizing a novel lactonization with inversion of stereochemistry | | | 40. The Chemistry and Biology of Alkannin, Shikonin and Related Naphthazarin Natural Products.
Papageorgiou, V. P.; Assimopoulou, A. N.; Couladouros, E. A.; Hepworth, D.; Nicolaou, K. C.Angew. Chem. Int. Ed.38, 270-300,1999.[Full Article][Project infos] | 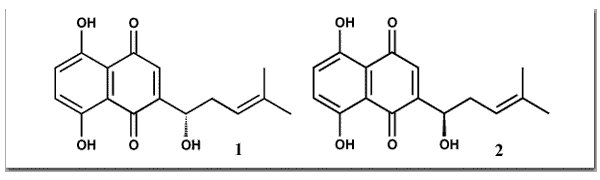 | | Abstract: There can be few natural products with histories as rich as those of the enantiomeric naphthoquinones alkannin and shikonin. Extracts from the roots of Alkanna tinctoria in Europe and Lithospermum erythrorhizon in the Orient have been used independently for many countries as natural red dyes and as crude drugs with the magic property of accelerating wound healing. This review is an attempt to tell the “whole story” of alkannin and shikonin, from the 5th century BC and presents up to date aspects of plant biology, pharmacology, and bioorganic, synthetic, and medicinal chemistry | | | 41. Inhibition of Topoisomerase I by Naphthoquinone Derivatives.
Plyta, Z. F.; Li, T.; Papageorgiou, V. P.; Mellidis, A. S.; Assimopoulou, A. N.; Pitsinos, E. N.; Couladouros, E. A.Bioorg. Med. Chem. Lett.8,3385-3390,1998.[Full Article] | 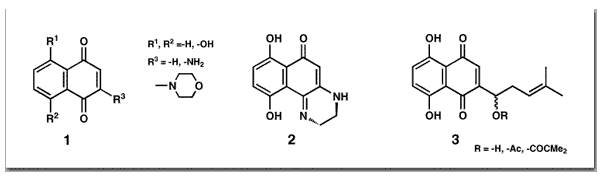 | | Abstract: Alkannin and shikonin are naturally occurring naphthoquinones. We have tested several derivatives of the title compounds and we have found that naphthoquinones bearing at least one phenolic hydroxyl group are potent inhibitors of topoisomerase I. The ability of the tested compounds to complex Zn++ parallels with a few exceptions their topoisomerase I inhibition properties while their intercalation and redox properties do not | | | 43. Total Synthesis of Combretastatins D.
Couladouros, E. A.; Soufli, I. C.; Moutsos, V. I.; Chadha, R. K. Chem. Eur. J.4,33-43,1998.[Full Article] | 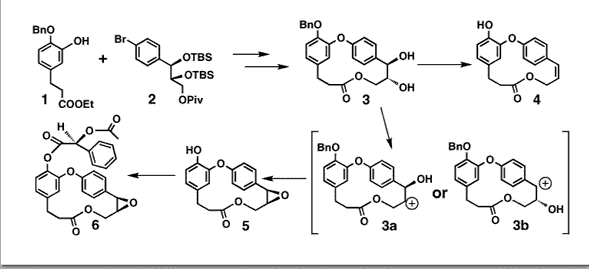 | | Abstract: The 15-membered caffrane ring of the natural product group of combretastatins D is synthesized in high yield with suitably functionalized saturated seco acids. The key step is a Mitsunobu-type macrolactonization. A common synthon is used for the construction of both combretastatins. The synthesis of combretastatin D-2 is completed by the use of Sammuelson’s dehydroxylation protocol. The asymmetric epoxide of combretastatin D-1 is constructed in two separate operations: one asymmetric center is fixed at an early stage of the synthetic route by Sharpless AD of a trans-styrene derivative, inducing the intramolecular formation of the asymmetric epoxide at the final stages. The synthesis of title compounds is accomplished in high overall yields (37% for D-1 and 41% for D-2, 9 steps in both cases). X-ray crystallographic analysis of the (S)-(+)-acetylmandelic ester of (–)-combretastatin D-1 verified its revised structure | | | 44. Asymmetric Synthesis of Alkannin and Shikonin.
Couladouros, E. A.; Plyta, Z. F.; Strongilos, A. T.; Papageorgiou, V. P.Tetrahedron Lett.38, 7263-7266,1997.[Full Article][Project infos] | 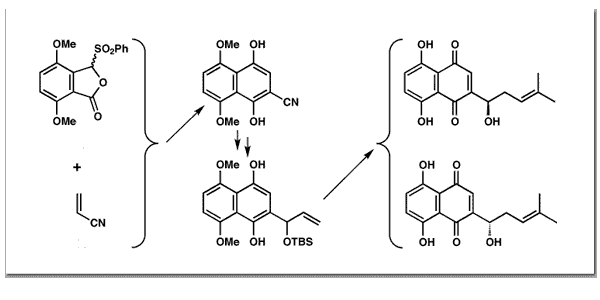 | | Abstract: A new, general and convergent route for the synthesis of the title compounds is presented. The polyoxygenated aromatic ring system is annulated in one operation by the condensation of a Michael type acceptor with an 1,4 dipole equivalent. The chiral center of the target is introduced via an asymmetric ally boration in high ee. Overall, the fully protected natural product is constructed within 8 steps in 35% total yield | | | 45. Efficient Synthesis of Aminonaphthoquinones and Azidobenzohydroquinones: Mechanistic Considerations of the Reaction of Hydrazoic Acid with Quinones. An Overview.
Couladouros, E. A.; Plyta, Z. F.; Haroutounian, S. A.; Papageorgiou, V. P.J. Org. Chem.62, 6-10,1997.[Full Article] | 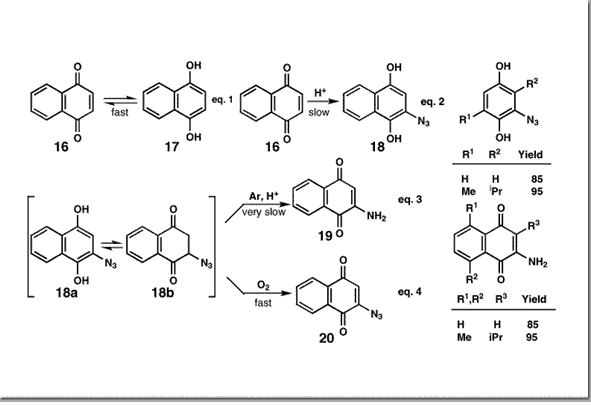 | | Abstract: Parameters useful to predict and control the reaction outcome of conjugate addition of hydrazoic acid to quinines have been studied, and the optimum conditions for the efficient synthesis of aminonaphthoquinones and azidobenzohydroquinones are reported. The application of this reaction for the efficient formal synthesis of dephostatin is also presented | | | 50. A General Procedure for the Efficient Synthesis of Alkylamino)naphtho-quinones.
Couladouros, E. A.; Plyta, Z. F.; Papageorgiou, V. P.J. Org. Chem.61,3031-3033,1996.[Full Article] | 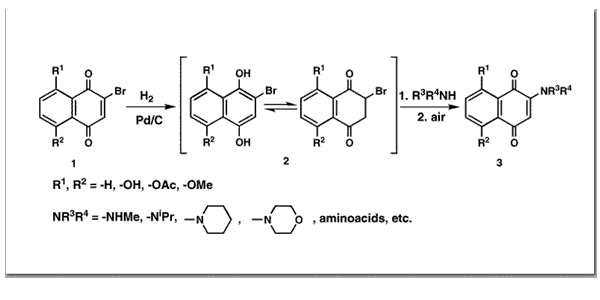 | | Abstract: In the present work we try to investigate the nucleophilic substitution of naphthoquinones. As a substrate for our study we have chosen bromonaphthoquinone 1 which can be easily reduced to compound 2. The latter is in equilibrium between the enol- and the keto-form, the position of the equilibrium being dependent on the substitution pattern. In contrast to the oxidized state 1, the keto-form of compound 2 possesses a simple, very reactive electrophilic carbon, i.e. the bromo-substituted one. Accordingly, after treatment of 1 with H2 over Pd/C catalyst and subsequent addition of an amine, a fast and clean substitution took place. Additional exposure of the reaction mixture to atmospheric oxygen afforded the observed amino derivative 3 in high yield as the isolation and purification of the product in most cases is performed merely by recrystallization | | | 52. Total Synthesis of Natural (-)-Combretastatin D-1.
Couladouros, E. A.; Soufli, I. C.Tetrahedron Lett.36,9369-9372,1995.[Full Article] | 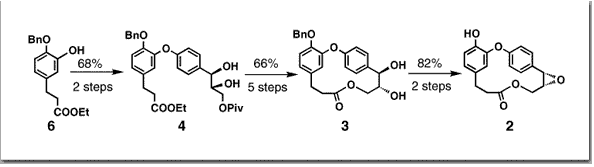 | | Abstract: Combretastatins D-2, and D-1, are chemically classified as caffrane macrolactones and show PS cell line activity corresponding to ED50 5.2 and 3.3 ?g/ml respectively. In conjunction with our interest in the total synthesis and the comparative evaluation of medium sized macrolactones, we present herein the first total synthesis of naturally occurring (-)-Combretastatin D-1 via a 12 step sequence. Sharpless asymmetric dihydroxylation was used in order to introduce the appropriate asymmetry. Regiospecific cyclodehydration of the resulting diol gave the title compound in 96% ee | | | 53. Total Synthesis of Brevetoxin B. 2. Second Generation Strategies and Construction of the Dioxepane Region [DEFG].
Nicolaou, K. C.; Theodorakis, E. A.; Rutjes, F. P. J. T.; Sato, M.; Tiebes, J.; Xiao, X.-Y.; Hwang, C.-K.; Duggan, M. E.; Yang, Z.; Couladouros, E. A.; Sato, F.; Shin, J.; He, H.-M.; Bleckman, T.J. Am. Chem. Soc.117, 10239-10251,1995.[Full Article][Project infos] | 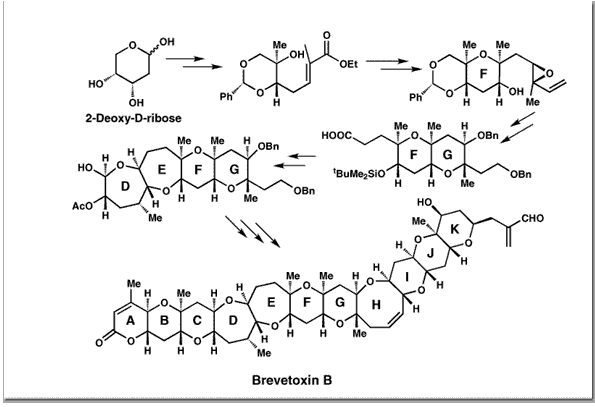 | | Abstract: In this paper a number of second generation strategies toward brevetoxin B are described. The main themes of these studies were developed around a retrosynthetic analysis which defined suitable ABCDEFG and IJK ring systems as potential advanced intermediates for a convergent strategy and projected the oxocene ring system as the last ring to be closed. A successful synthesis of the DEFG ring framework, a precursor to the larger ABCDEFG ring system, was developed. Several methods for the elaboration of the latter compound to a more advanced intermediate were also explored. A number of new tactics and strategies were also developed. Among them, a convergent Cr/Ni-promoted coupling procedure of lactone-derived enol triflates and aldehydes proved quite general and applicable to a potential precursor of the ABCDEFG ring system of brevetoxin B | | | 54. Chemical Synthesis and Biological Evaluation of C-2 Taxoids.
Nicolaou, K. C.; Renaud, J.; Nantermet, P. G.; Couladouros, E. A.; Guy, R. K.; Wrasidlo, W.J. Am. Chem. Soc.117, 2409-2420,1995.[Full Article][Project infos] | 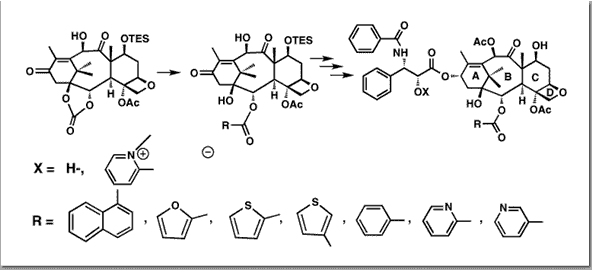 | | Abstract: An efficient, general method for the synthesis of 1,2-hydroxy esters by regioselective nucleophilic opening of 1,2-cyclic carbonate systems has been developed. A reliable and practical route giving access to the taxoid carbonate from the readily available 10-deacetylbaccatin III has been established. Nucleophilic opening of the carbonate with a variety of nucleophiles provided a number of novel Taxols. Water-soluble taxoid onium salts were also synthesized and studied. A selected number of taxoids were subjected to cytotoxicity and tubulin polymerization assays as well as in vivo studies. The results of these studies are reported herein | | | 56. Total Synthesis of Taxol. 1. Retrosynthesis, Degradation and Reconstitution.
Nicolaou, K. C.; Nantermet, P. G.; Ueno, H.; Guy, R. K.; Couladouros, E. A.; Sorensen, E. J.J. Am. Chem. Soc.117, 624-633,1995.[Full Article][Project infos] | 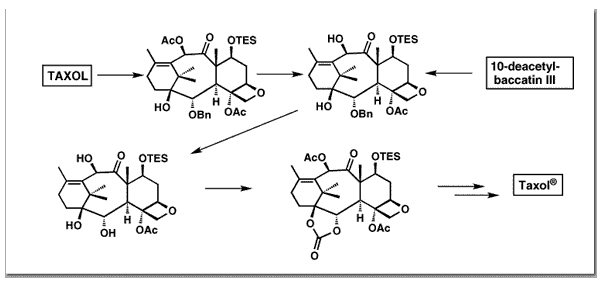 | | Abstract: A successful strategy for the enantioselective synthesis of the natural stereoisomer of Taxol has been developed. This strategy utilized the convergent assembly of Taxol’s central eight-membered ring from performed synthons for the six-membered rings followed by late introduction of the oxetane ring and side chain. Degradative studies confirmed the viability of certain crucial manipulations including allylic oxidation of the C-13 position and regioselective introduction of the C1-hydroxyl, C2-benzoyloxy moiety. Additionally, a convenient method for the large-scale production of the 5-membered ring carbonate intermediate, a derivative useful for C2 analog production via nucleophilic opening with a variety of reagents, was developed | | | 58. Synthesis of C-2 Taxol Analogues.
Nicolaou, K. C.; Couladouros, E. A.; Nantermet, P. G.; Renaud, J.; Guy, R. K.; Wrasidlo, W.Angew. Chem., Int. Ed. Engl.33,1581-1583,1994.[Full Article][Project infos] | 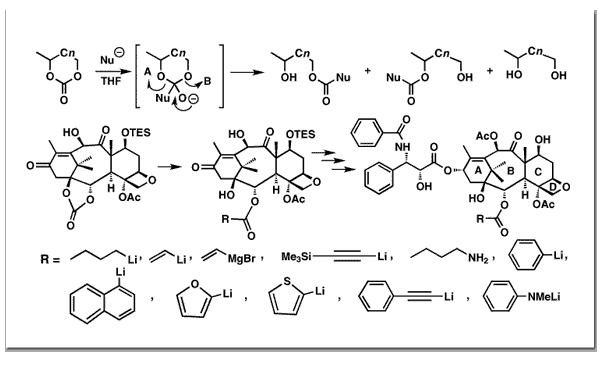 | | Abstract: In this communication we report a general method for the regioselective functionalization of cyclic carbonates by nucleophilic ring opening and its application in the synthesis of a series of new taxoids functionalized at C-2; a family of compounds hitherto difficult to obtain. Preliminary biological evaluation of selected compounds of this series revealed cytotoxicities comparable to that of taxol for the thiophene and the furanyl analogue and less than that of taxol for the p-dimethylaminophenyl taxoid. These results suggest rather well-defined demands by the taxol receptor for the C-2 ester group | | | 59. Synthesis of Combretastatin D-2..
Couladouros, E. A.; Soufli, I. C.Tetrahedron Lett.35,4409-4412,1994.[Full Article] | 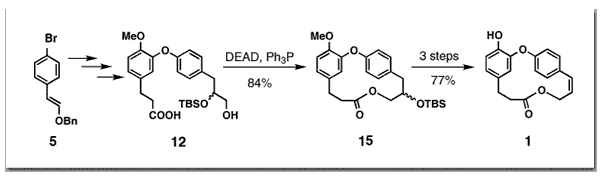 | | Abstract: An Efficient Route to Caffrane Macrolactones Combretastatin D-2, a biaryl derivative isolated from the South African folk medical tree Combretum caffrum, was synthesized via a 10 step sequence. Methods reported to date concerning the synthesis of this kind of medium sized rings suffer from the low to moderate yields during the macrolactonization step. In this report the general aspect is to perform the macrolactonization on a saturated substrate (relying on MM3 calculations for the strain energy of the ring) and then to restore the unsaturation via dehydrohalogenation at a latter step. As a result we develop an effective route for the synthesis of the title compound and a concise synthetic plan which can be utilized to rapidly prepare a variety of related analogues | | | 62. Total Synthesis of ?axol.
Nicolaou, K. C.; Yang, Z.; Liu, J. J.; Ueno, H.; Nantermet, P. G.; Guy, R. K.; Claiborne, C. F.; Renaud, J.; Couladouros, E. A.; Paulvannan, K.; Sorensen, E. J.Nature 367, 630-634,1994.[Full Article][Project infos] | 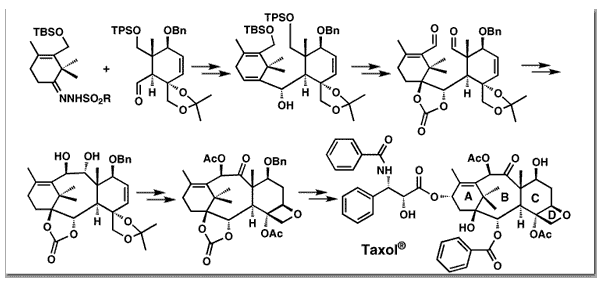 | | Abstract: The scarcity of taxol and the ecological impact of harvesting it have prompted extensive searches for alternative sources including semisynthesis, cellular culture production and chemical synthesis. The latter has been attempted for almost two decades, but these attempts have been thwarted by the magnitude of the synthetic challenge. Here we report the total synthesis of taxol by a convergent strategy, which opens a chemical pathway for the production of both the natural product itself and a variety of designed taxoids. Thus, in the synthetic direction the following key operations were proposed: i) two fragments representing precursors to rings A and C, were to be coupled by a Shapiro reaction and a McMurry coupling to assemble the ABC ring skeleton, ii) instalment of the oxetane ring, iii) addition of the various substituents around the peripheries of rings B and C, iv) oxygenation at C-13 and v) esterification to attach the side chain | | | 63. Synthesis of Novel Taxoids.
Nicolaou, K. C.; Claiborne, C. F.; Nantermet, P. G.; Couladouros, E. A.; Sorensen, E. J.J. Am. Chem. Soc.116, 1591-1592,1994.[Full Article][Project infos] | 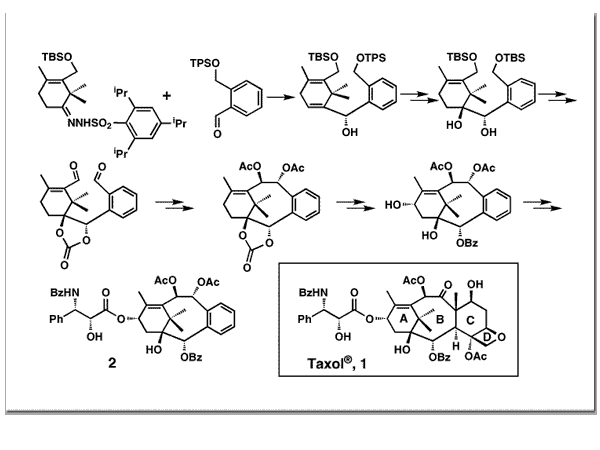 | | Abstract: Taxol, a naturally occurring substance isolated from the Pacific yew tree (Taxus brevifolia) has recently been approved for clinical treatment of cancer patients. Its chemical synthesis and the synthesis of taxoid model systems have been the focus of extensive research efforts. Recent publications from these laboratories disclosed a convergent approach to the taxoid family of compounds in which a Shapiro-type coupling is used as a means of joining rings A and C. In this communication we describe the application of this strategy to the synthesis of advanced taxoid systems containing the intact AB ring framework of taxol, including the side chain, and in which the CD ring system is replaced with an aromatic moiety. Furthermore, this novel synthetic procedure will later be used to the total synthesis of Taxol and various derivatives | | | 66. Synthesis and Antimicrobial Properties of 2H-Pyran-3(6H)-one Derivatives and Related Compounds.
Georgiadis, M. P.; Couladouros, E. A.; Delitheos, A. K.J. Pharm. Sci.81,1126-31,1992.[Full Article] | 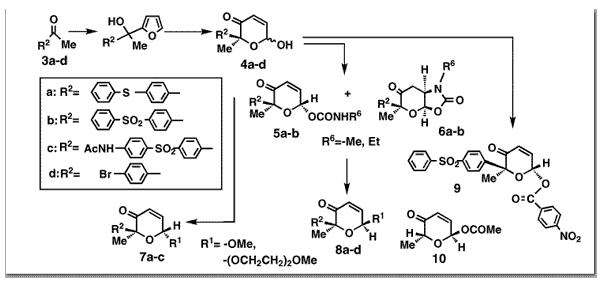 | | Abstract: The synthesis of several derivatives of 2H-pyran-3(6H)-ones and their Michael adducts is described. Phenylthio, benzenesulfonyl, p-acetylaminobenzenesulfonyl and p-bromophenyl substituents are beneficial for activity against gram-positive bacteria. 2-[4-(Phenylthio)phenyl]-2-methyl-6-methoxy-2H-pyran-3(6H)-one (8a) showed a minimum inhibitory concetration of 1.56 ?g/ml against Staphylococcus aureus ATCC 2593, and 2-[4-(phenylthio)phenyl]-2-methyl-6-[(p-nitrobenzoyl)oxy]-2H-pyran-3(6H)-one showed a minimum inhibitory concetration of 0.75 ?g/ml against Streptococcus sp. C203M. In general, derivatives of 6-hydroxy-2H-pyran-3(6H)-ones with substituents at C-2 and C-6 showed significant activity against gram-positive bacteria. More specifically, the bulkier the C-2 substituent, the greater the antibacterial activity. Michael adducts of thiols showed activity, which may be due to a retro-Michael reaction. In conclusion, the ?,?-enone system is essential for the activity of 6-hydroxy-2H-pyran-3(6H)-ones, and the size and nature of substituents at C-2 are associated with antimicrobial activity | | | 74. Bridging of Macrodithionolactones to Bicyclic Systems. Synthesis and Modeling of Oxapolycyclic Frameworks.
Nicolaou, K. C.; Hwang, C.-K.; Marron, B. E.; DeFrees, S. A.; Couladouros, E. A.; Abe, Y.; Carroll, P. J.; Snyder, J. P.J. Am. Chem. Soc.112, 3040-3054,1990.[Full Article][Project infos] | 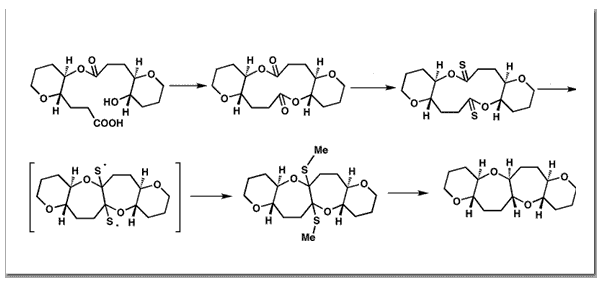 | | Abstract: A new reaction involving bridging of macrodithionolactones to bicyclic systems is described. A series of macrodiolides was prepared and converted to the requisite macrodithionolactones. The latter substrates were induced to undergo bridging across the macrocyclic ring by exposure to sodium naphthalenide, leading to stable bicyclic systems upon addition of methyl iodide. The mixed thioketals so obtained were converted to a number of saturated or unsaturated bicyclic or polycyclic systems by removal of the sulfurs. The stereochemistry of bridging follows the relative energy of the cis and trans products rather than the conformational preferences of the macrocycles. This is confirmed by MM2 calculations and X-ray crystal structure determinations. The unusual stereochemical course of some of the reported reactions, elucidated by X-ray, has been given a mechanistic basis by conformation searching coupled by energy evaluation by MM2 and PRDDO. Several new sets of MM2 parameters were evolved during this work | | | 76. Products from Furans IV. Selective Oxidation of 2-Furfuryl Alcohol Derivatives, in the Presence of Aryl Thioethers, with N-Bromosuccinimide (NBS).A New Procedure for the Preparation of 2H-Pyran-3(6H)-ones.
Georgiadis, M. P.; Couladouros, E. A.J. Org.Chem.51,2725-2727,1986.[Full Article] | 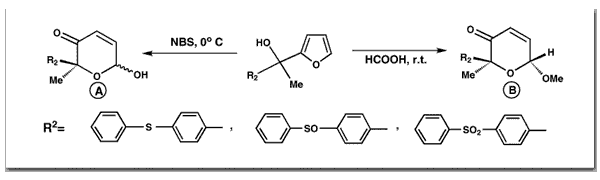 | | Abstract: A new general fast method (as fast as a titration) for converting 2H-furfuryl alcohols to 2H-pyran-3(6H)-ones by the use of NBS as an oxidant is presented. NBS oxidizes selectively the furfuryl alcohols without affecting thioether substituents. This novel oxidation procedure is simple, gives high yields, and is considerably cheaper than the MCPBA method. By the use of NBS method, we were able to synthesize 6-hydroxy-2-[p-(phenylthio)phenyl)]-2-methyl-2H-pyran-3(6H)-one and 6-hydroxy-2-[p((phenylthio)oxy)phenyl]-2-methyl-2H-pyran-3(6H)-one which were target molecules for new anticoccidials and which could not be synthesized by the use of other oxidative agents | | | 78. Products from Furans III. Crystal and Molecular Structure, Proton Nuclear Magnetic Resonance, and Conformational Studies of 2-Aryl Substituted 2-Methyl-6-hydroxy-2H-pyran-3(6H)-one Derivatives.
Georgiadis, M. P.; Couladouros, E. A.; Polissiou, M. G.; Filippakis, S. E.; Mentzafos, D.; Terzis, A.J. Org.Chem.47,3054-3058,1982.[Full Article] | 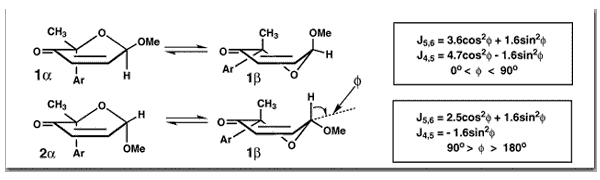 | | Abstract: Derivatives of 2H-pyran-3(6H)-ones have been reported as antimicrobials and coccidiostatics as well as intermediates in the synthesis of sugars, disaccharides, maltol, and biological metabolites. We have synthesized some 2H-pyran-3(6H)-ones having a 2-(para-substituted phenyl) substituent in an effort to produce compounds with increased antimicrobial and coccidiostatic activities and at the same time to investigate the configuration of this class of compounds which heretofore has not been examined. We note that the nature and the number of substituents at C2 determine the configuration of the predominant diastereoisomer and that the Garbisch equation cannot be applied to this type of molecules because of the axially aryl group. Finally, a direct configurational assignment is possible from the quotient J5,6/J4,6 of vicinal to allylic coupling constants | | | |